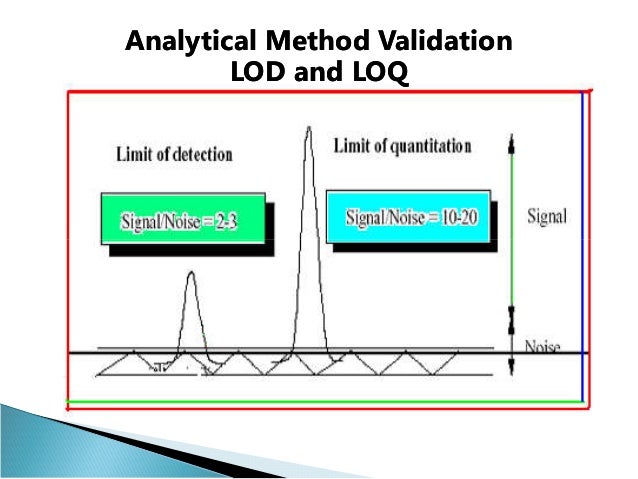
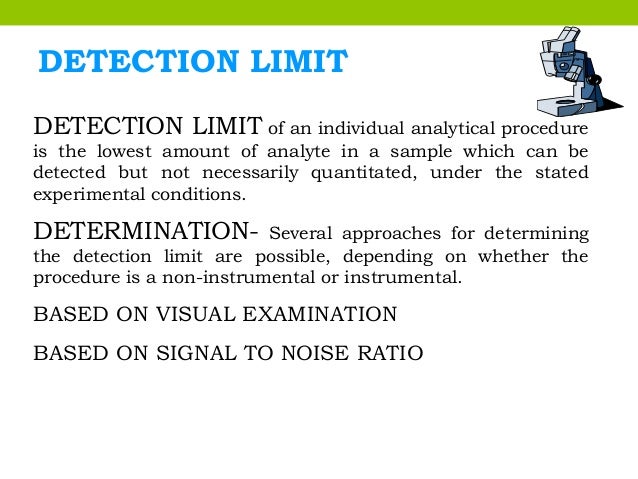
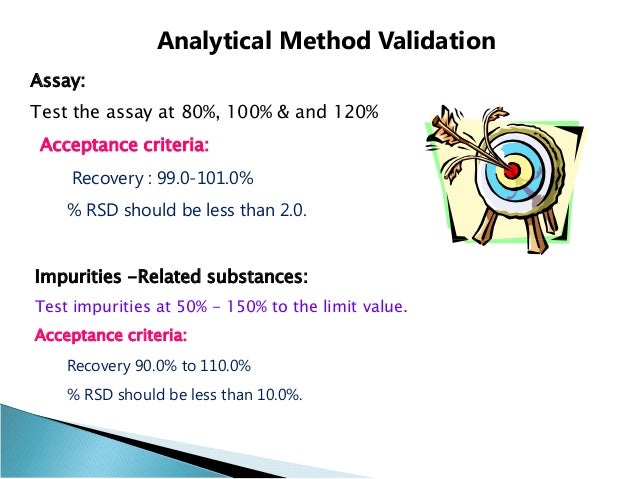
Analytical Test Method Validation Protocol Template. Reporting. • Analytical validation demonstrates the accuracy, precision, reproducibility of the test- how well does the test measure what it claims to measure? • The goals for demonstrating analytical and clinical validation are no different for MS or multi-analyte arrays than they are for any other test. Limit tests for the control of Impurities; Quantitative tests of the active moiety in samples of the drug substance or drug product or other selected component(s) in the.

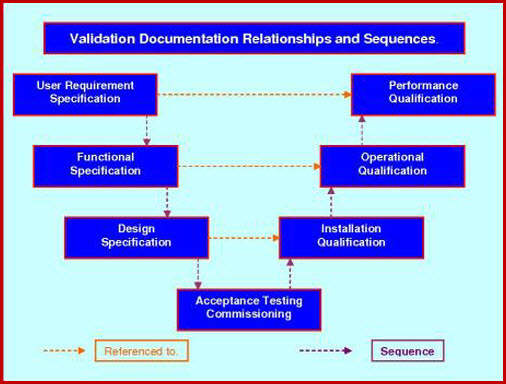
Process Validation (SOP & Protocol), An establishing documented evidence through collection and evaluation of data from process design stage to Validate the Analytical methods for in process testing and finished product analysis.
This method validation protocol applies to all test methods performed for release or stability evaluation of all strengths of Ciprofloxacin Tablets.
Methods Validation: Establishing documented evidence that provides a high degree of assurance that a HC has also issued templates recommended as an ap- proach for summarizing analytical Writing a Test Method Validation Protocol. PPT - Analytical Method Validation Definition and Protocol PowerPoint presentation After you enable Flash, refresh this page and the presentation should play.