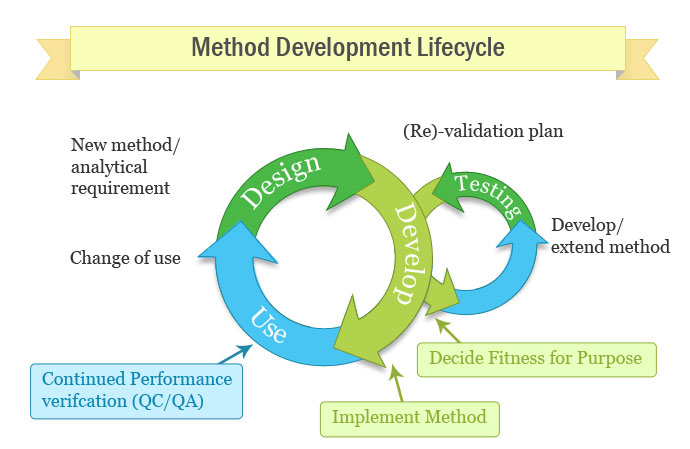
Analytical Method Validation Protocol Sample. Steps in Method Validation: Develop a validation protocol or operating procedure for the Advantages of Analytical Method Validation: The biggest advantage of analytical method CONCLUSION: Analytical method validation and method transfer data playing a fundamental role. Evaluation of the method development with the sample.
![Analytical method validation raaj gprac [compatibility mode]](https://image.slidesharecdn.com/analyticalmethodvalidationraajgpraccompatibilitymode-120830120141-phpapp01/95/analytical-method-validation-raaj-gprac-compatibility-mode-19-728.jpg?cb=1346328139)
To ensure the accuracy and permanency of collected data.
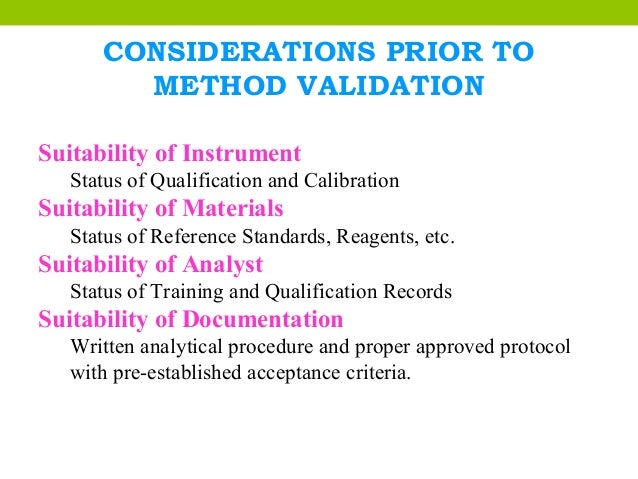
The process should follow a validation protocol, also considering instruments, supplies and reagents.
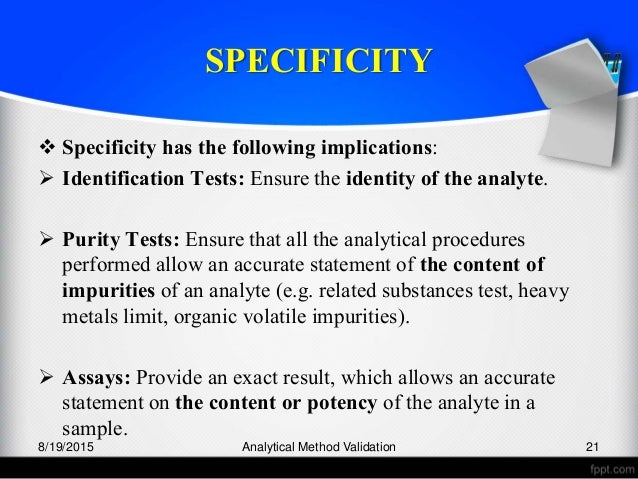
Establishing documented evidence which provides a high degree of assurance that a specific process (analytical test method) will consistently produce a. A comprehensive guide to validating analytical methods for pharmaceutical analysis. Validation is the process of determining the performance characteristics of a method/procedure or It is a prerequisite for judgement of the suitability of produced analytical data for the intended use.